
MSAE 530
Binary Phase Diagram III

2
Overview of this lecture
• Introduce some complicated phase
diagrams
* Components with different crystal structures
* Polymorphism
* Stoichiometric compound formation
* Intermediate phases

3
Introduction
Some complicated phase diagrams:
• Two metal components have different crystal structure but do
not form compounds.
• One of the metal components has more than one crystal
structure and undergoes a polymorphic transformation in a p, T
range of interest.
• Stoichiometric compounds are formed between the two metal
components.
•Non-stoichiometric intermediate phases are formed.
4
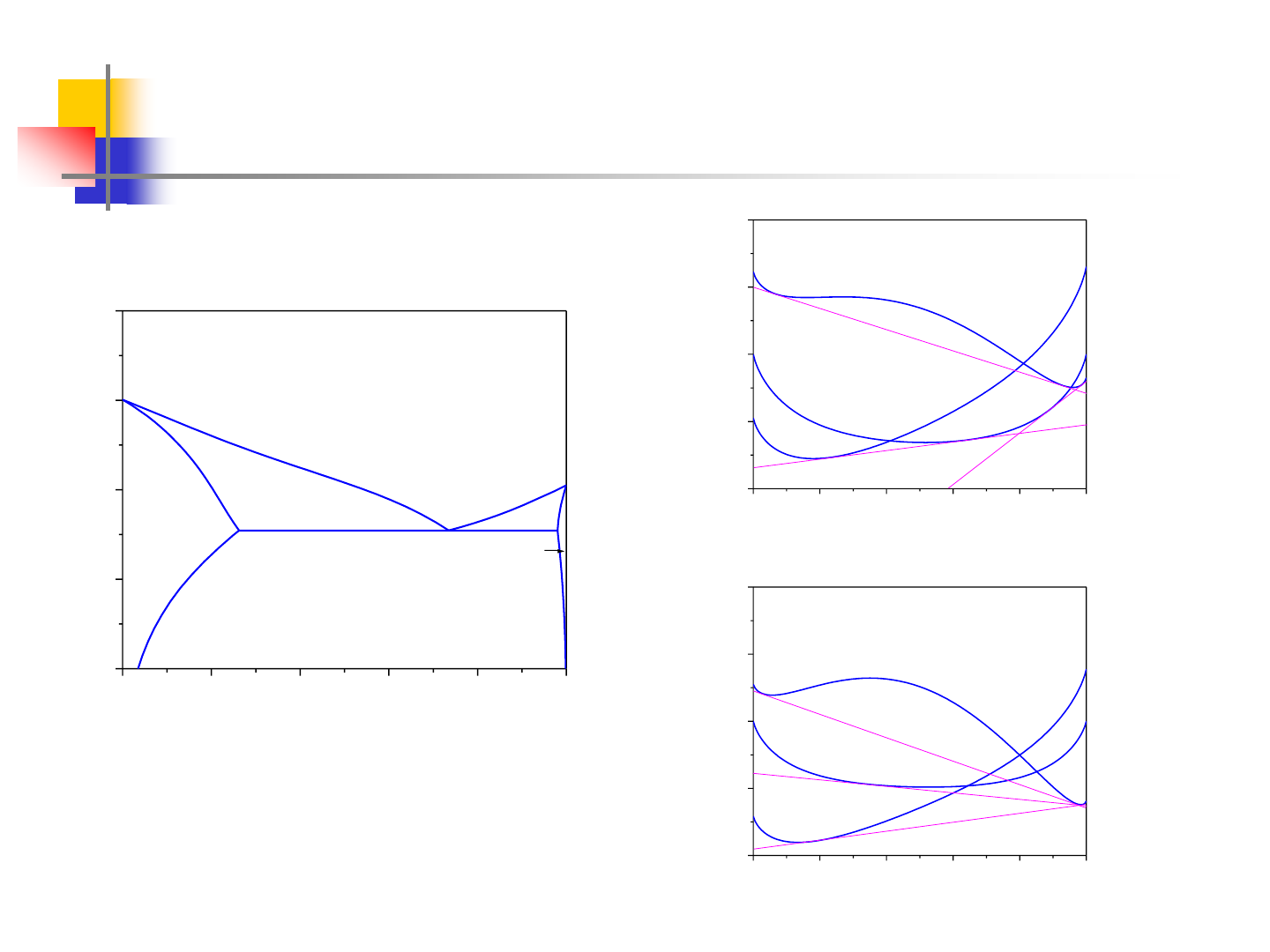
Components with Different Crystal Structures
0.0 0.2 0.4 0.6 0.8 1.0
300
400
500
600
700
T
E
=456K
(Sn)
(Pb)
L
Sn
Pb
Temperature, K
Mol. Fracn. Sn
0.0 0.2 0.4 0.6 0.8 1.0
-2000
-1000
0
1000
2000
Liquid
T>T
E
Sn
Pb
G(J/mol)
x
2
0.0 0.2 0.4 0.6 0.8 1.0
-2000
-1000
0
1000
2000
Liquid
T<T
E
Sn
Pb
G(J/mol)
x
2
A(l) and B(l) are the reference states
5
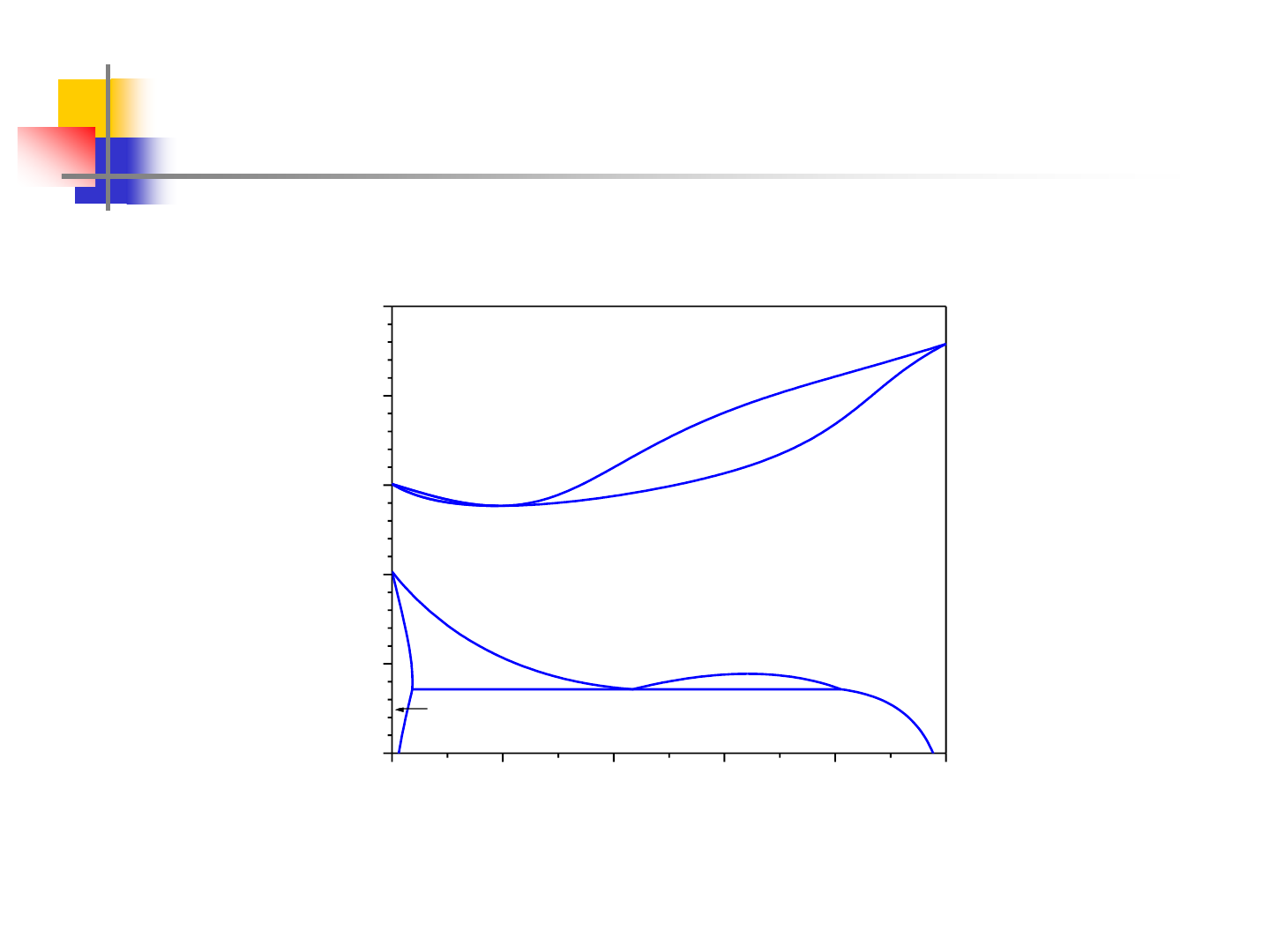
Polymorphism
0.0 0.2 0.4 0.6 0.8 1.0
1000
1500
2000
2500
3000
3500
2016K
Ta
Hf
(Hf,Ta)
(Hf,Ta)
Liquid
Temperature, K
Mol. Fracn. Ta
6
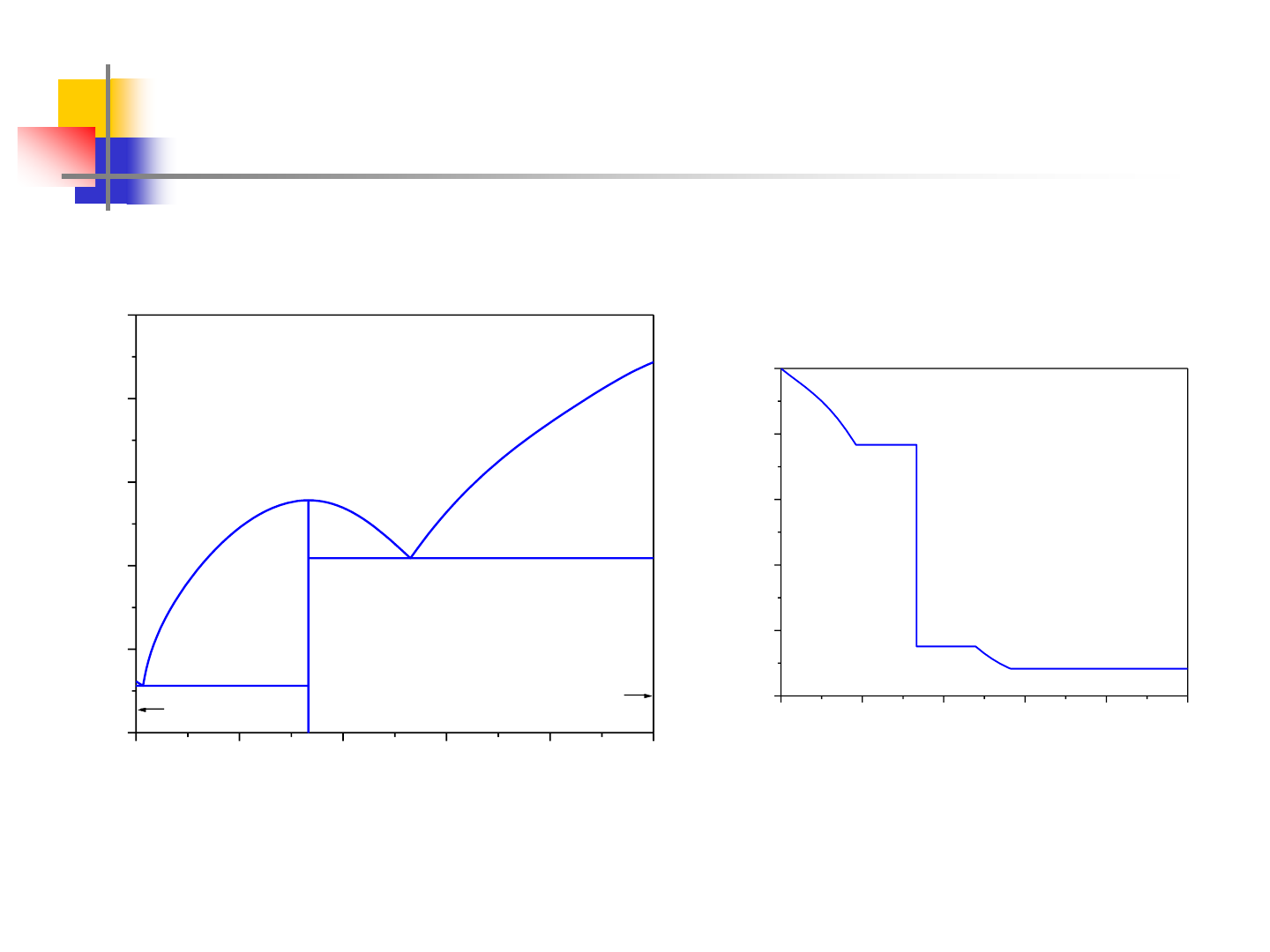
Stoichiometric Compound Formation
0.0 0.2 0.4 0.6 0.8 1.0
800
1000
1200
1400
1600
1800
Mg
2
Si
(Si)
(Mg)
Liquid
Si
Mg
Temperature, K
Mol. Fracn. Si
0.0 0.2 0.4 0.6 0.8 1.0
0.0
0.2
0.4
0.6
0.8
1.0
T=1275K
Mg
2
Si
Liq
Liq+Si
Liq+Mg
2
Si
Liq+Mg
2
Si
Liq
Si
Mg
a
Mg
rel. to Mg(
l)
Mol. Fracn. Si
7
Stoichiometric Compound Formation
)BA(G), x(x), x()x1(
11
xx11B11A1 −
=+− ss
)]B(Gx)A(G)x1[(
)T,s,BA(G)BA(G
mfus1mfus1
nmf
nm
1
xx1mr
11
+−−
=
+
−
)BA(G), x(x), x()x1(
11
xx12B12A1 −
=+− ll
)T,s,BA(G)x,(G)BA(G
nmfus
nm
1
1mixxx1mr
11
+=
+
−
l
)BA(S}TT{)T,BA(G
nmfusfusnmfus
− )BA(S}TT{)T,BA(G
nmfusfusnmfus
−
8
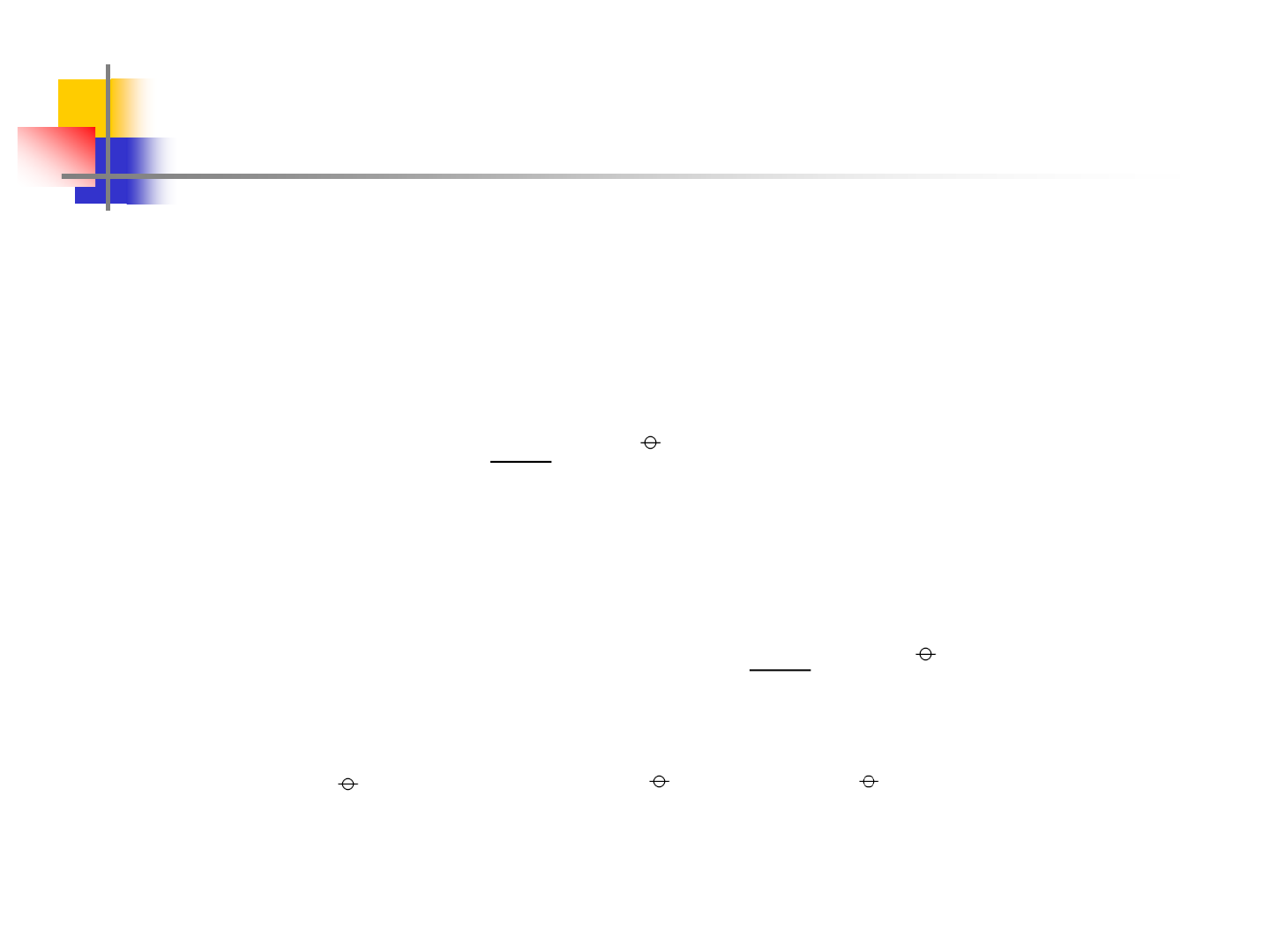
Intermediate Phases
0.0 0.2 0.4 0.6 0.8 1.0
400
600
800
1000
1200
1400
1600
1800
2000
2200
Tc
(Cr)
(Fe,Fe)
(Fe)
Liquid
Cr
Fe
Temperature, K
Mol. Fracn. Cr

9
END !