199
7 PanSolidification
PanSolidification is a module of Pandat
TM
software designed to simulate
solidification behavior under a variety of conditions with different cooling rates.
It is an extension of the Scheil model taking into consideration of back
diffusion in the solid, secondary dendrite arm coarsening, and the formation of
eutectic structure.
It is seamlessly integrated with the user-friendly Pandat
TM
Graphical User
Interface (PanGUI) as well as thermodynamic calculation engine, PanEngine.
The implementation of PanEngine guarantees reliable input data, such as
chemical potential, phase equilibrium and mobility. Figure 7.1 shows an
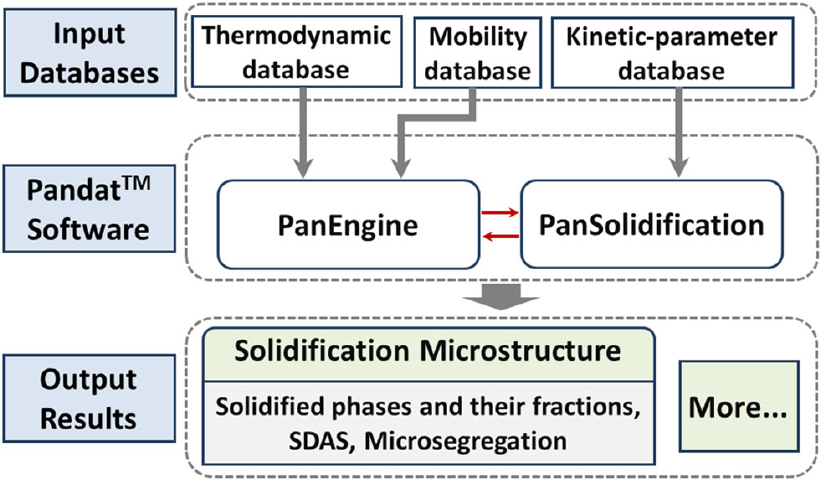
overall architecture of the PanSolidification module.
Figure 7.1 An overall architecture of the PanSolidification module
200
7.1 Features of PanSolidification
7.1.1 Overall Design
➢ The system composition profile, phase fraction, and phase concentration
evolution during solidification
➢ Secondary dendrite arm spacing (SDAS) evolution during solidification.
➢ Back diffusion during the entire solidification process.
7.1.2 Data Structure
Thermodynamic and mobility parameters are stored in TDB file, and the kinetic
parameters for undercooling and coarsening effects are stored in an SDB file in
“Extensible Markup Language” (XML) format, which is a standard markup
language and well-known for its extendibility. In accordance with the XML
syntax, a set of well-formed tags are specially designed to define the back
diffusion model for the morphology of primary phase and its corresponding
model parameters such as interfacial energy, latent heat, coarsening geometric
factor, dendrite tip factor, solute trapping parameter, solid diffusivity factor and
boundary layer factor.
7.1.3 Numerical Model
The PanSolidification module, which is developed by coupling a solidification
micro-model with PanEngine, is basically a modified Scheil model incorporating
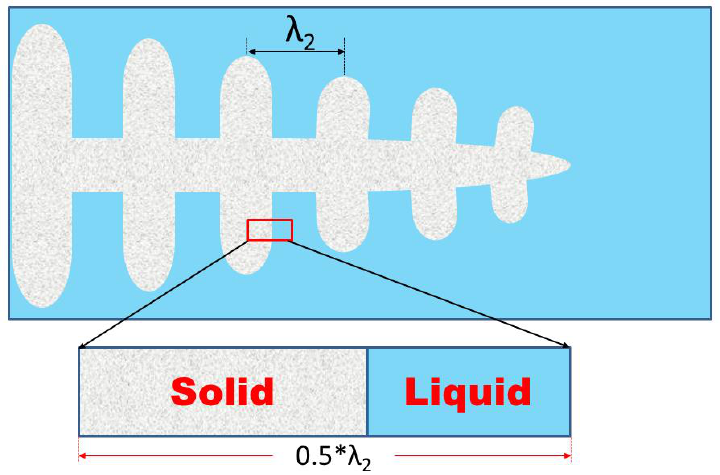
back-diffusion, undercooling, and dendrite arm coarsening. Figure 7.2 shows a
sketch of dendrite, with a big solid trunk as the primary dendrite arm and fine
secondary dendrite arms symmetrically distributed at the sides; the SDAS is
indicated as λ
2
. A one-dimensional morphology within the interdendritic region
of secondary arms is usually used to describe the solidification processing (as
enlarged and shown at the bottom part of Figure 7.2). Because of the symmetry
of the dendrite arms, there is no mass flow through the arm center. Therefore,
only one half of the arm spacing is considered.
201
Figure 7.2 A schematic diagram of dendrites in the solid and liquid region
7.1.3.1 Back diffusion in the solid
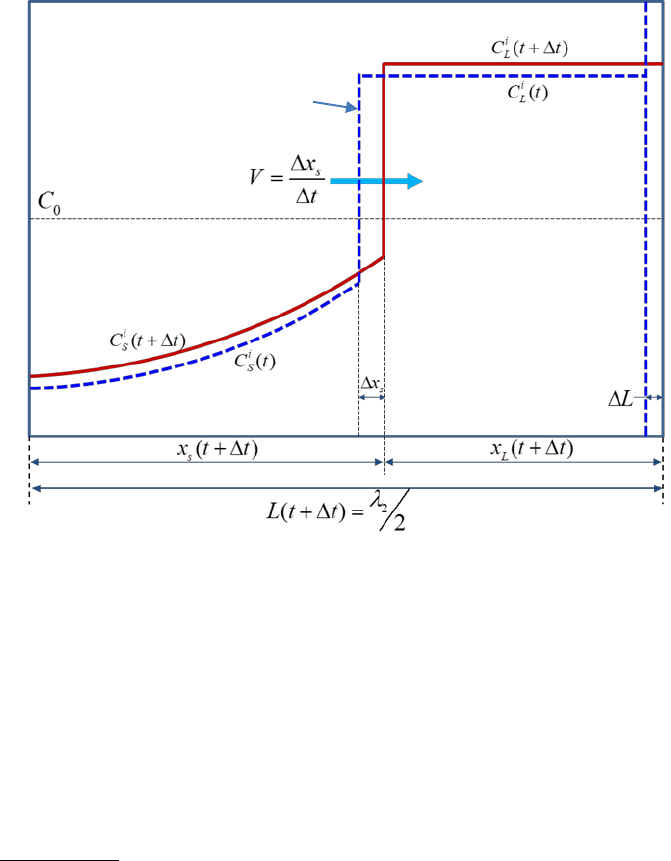
The evolution of the concentration profile for component i in the considered
dendrite arm is shown schematically in Figure 7.3.
i
L
C
and
i
S
C
are compositions
of component i within the liquid and solid phases (given the unit of wt.% in this
work), respectively. V is the velocity of S/L interface. During the time interval Δt,
the S/L interface advances Δx
s
(due to solidification) and the length of the
solidification region increases by ΔL (due to the SDAS coarsening). For the
current solidification simulation at each time step, three major tasks are
carried out: (1) calculate the composition of each component at the S/L
interface including the undercooling effects and local-equilibrium conditions; (2)
solve the diffusion equations within the solid phase; (3) update the length scale
to conserve mass balance for every component. More detailed description on
the back diffusion can be found in some textbooks [1974Fle, 1985Kur].
202
Figure 7.3 A schematic plot showing the composition distribution of component
i in a dendrite arm at time t and t+Δt.
7.1.3.2 Micro-model for dendrite arm coarsening
The initial SDAS is about twice of the dendrite tip radius:
0
2
T
r
and
T
r
is
described as a function of initial alloy composition, growth rate, and
independent of temperature gradient
2
0
0
2
L
T
ef
DT
r
V T k H
=
(7.1)
where V, ΔT
0
, k
e
are the interface solidification velocity, freezing temperature
range, and equilibrium partition coefficient, respectively. δ is a constant being
dependent on the harmonic of the perturbation.
The dendrite arm spacing needs to be known since it sets the diffusion
distances in the liquid and solid phases. Owing to the re-melting and re-
solidification mechanism, dendrite arm coarsening contributes significantly to
homogenization during solidification. The calculation of coarsening is described
as below [1986Roo]:

203
33
0
0
t
gMdt
−=
(7.2)
0
is the initial SDAS obtained from the calculated dendrite tip radius as
described in above equation 7.2, and
is the model predicted SDAS at a
certain time. M is coarsening parameter which is proportional to
1/3
, t is time
and g is the geometry factor representing the influence of the dendrite geometry.
For a binary system, the coarsening parameter M is defined as [1990Roo]:
(1 )
L
vv
f L v L
DT
M
H m k C
=
−
(7.3)
For a multicomponent system, the coarsening parameter must be calculated
separately for each alloying element. Then, the following model is used to take
into consideration all the solute elements:
1
1
1/
n
j
j
M
M
=
=
(7.4)
All phase equilibrium related quantities needed in the above equations (such as
m
L
and k
e
) are directly calculated via PanEngine [2009Cao] at each time step by
assuming the local equilibrium at the liquid/solid interface.
7.1.4 The Solidification Kinetic Database Syntax and
Examples
The Solidification kinetic database (.SDB) uses the XML format, which defines
the back diffusion model for the morphology of primary phase and its
corresponding model parameters such as interfacial energy, latent heat,
coarsening geometric factor, dendrite tip factor, solute trapping parameter,
solid diffusivity factor, boundary layer factor, and so on.
In the SDB, a series of alloys can be defined. A sample SDB file is given below,
<Alloy name="Mg alloys">
204
<solvent name="Mg"/>
<primary_phase name="Hcp"/>
<ParameterTable name="">
<Parameter name="coordinate" value="0" description = "geometry of
dendrite. 0 for plate; 1 for cylinder; 2 for sphere" />
<Parameter name="interfacial_energy" value="0.065" description =
"interfacial energy, unit = J/m^2"/>
<Parameter name="latent_heat" value="5.5e8" description =
"latent heat, unit=J/m^3"/>
<Parameter name="solute_trapping_parameter" value="1e-9" description =
"solute trapping parameter, unit=m"/>
<Parameter name="sound_velocity" value="1000" description =
"sound velocity, unit=m/s"/>
<Parameter name="coarsening_geometric_factor" value="40" description =
"No unit"/>
<Parameter name="dendrite_tip_factor" value="1" description =
"No unit"/>
<Parameter name="solid_diffusivity_factor" value="0.2" description =
"No unit"/>
<Parameter name="boundary_layer_factor" value="1" description =
"No unit"/>
</ParameterTable>
</Alloy>
</sdb>
In this sample SDB, “Mg alloys” is defined as the name of the alloy, the primary
phase is thus set as “Hcp” phase. A set of parameters for each phase, such as
interfacial energy, latent heat and so on, can be defined in “ParameterTable”.
205
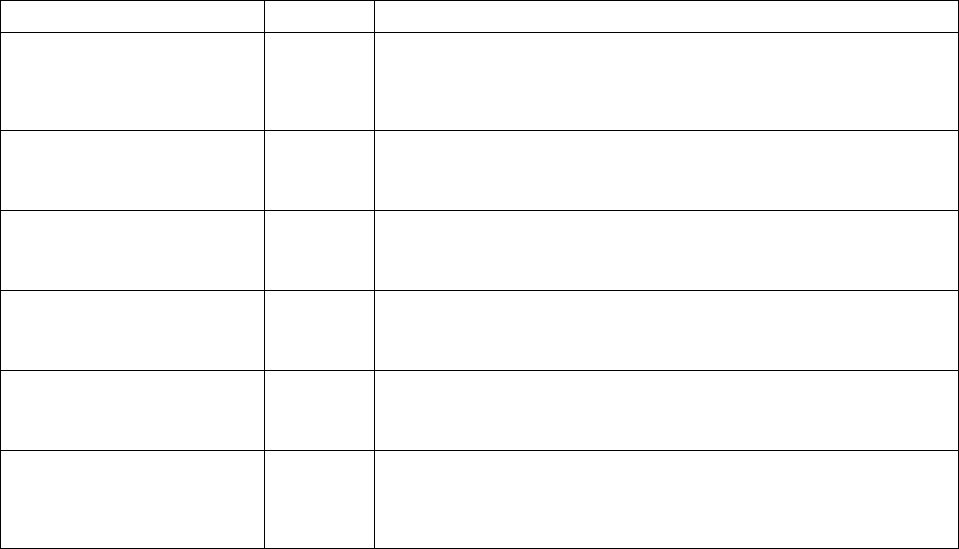
The kinetic model parameters that can be defined under “ParameterTable” are
listed in Table 7.1.
Table 7.1 Kinetic parameter models in sdb
Name
Unit
Description
Coordinate
N/A
Describe the geometry of dendrite for back diffusion model.
<Parameter name="coordinate" value="0" description =
"geometry of dendrite. 0 for plate; 1 for cylinder; 2 for
sphere" />
Interfacial_Energy
2
/Jm
Interfacial energy
<Parameter name="interfacial_energy" value="0.065"
description = "interfacial energy, unit = J/m^2"/>
Latent_heat
3
/Jm
Latent Heat of the alloy
<Parameter name="latent_heat" value="5.5e8" description =
"latent heat, unit=J/m^3"/>
Solute_Trapping_Parameter
N/A
Solute trapping parameter
<Parameter name="solute_trapping_parameter" value="1e-9"
description = "solute trapping parameter, unit=m"/>
Sound_velocity
N/A
Sound velocity
<Parameter name="sound_velocity" value="1000" description
= "sound velocity, unit=m/s"/>
Coarsening_Geometric_Factor
N/A
A factor adjusting adjust the coarsen speed of the dendrite.
<Parameter name="coarsening_geometric_factor" value="40"
description = "No unit"/>
206
7.2 Tutorial
7.2.1 Step 1: Create a PanSolidification Project
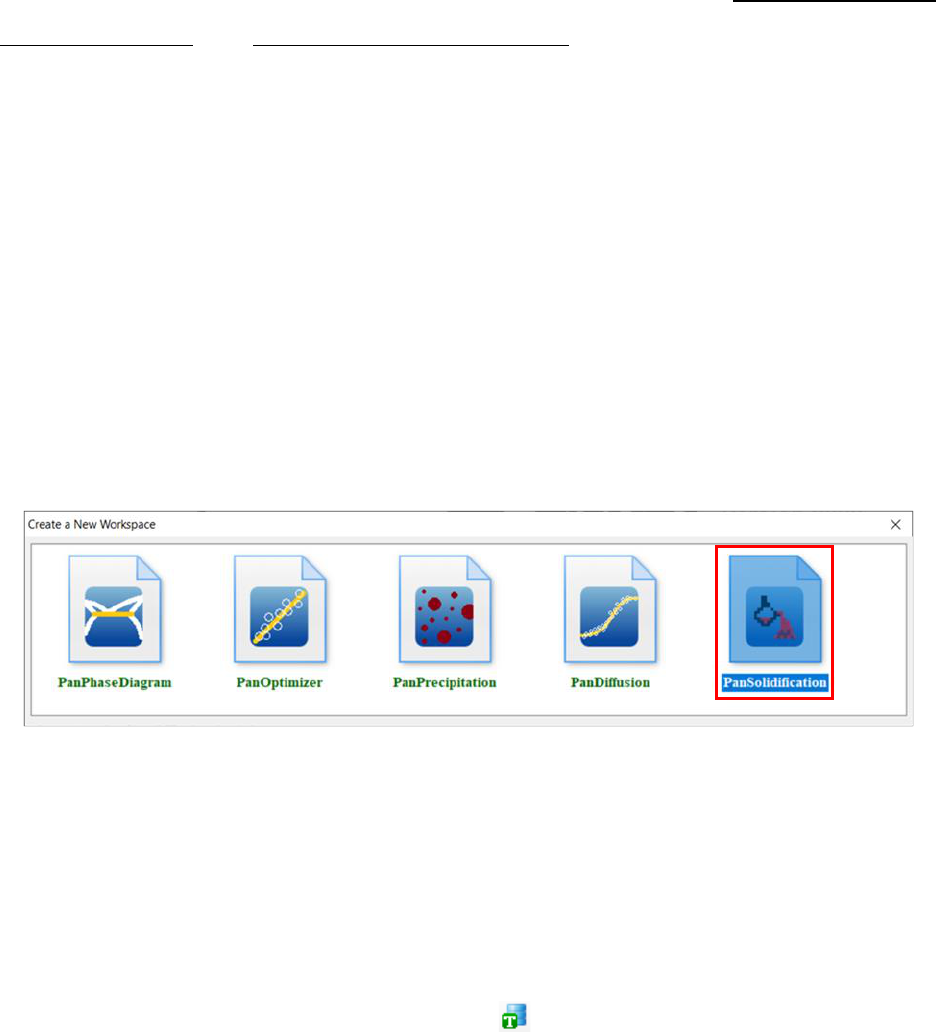
Users can create a PanSolidification project through menu “File → Create a
New Workspace” or “File → Add a New Project” in an existing workspace. The
“Module Window” pops out for user to choose a module for the new project as
shown in
Figure 7.4. Choose “PanSolidification” module for Solidification simulation, and
the PanSolidification project will be created after user click on Create button or
double click on the PanSolidification icon.
Figure 7.4 Creating a PanSolidification workspace
7.2.2 Step 2: Load Thermodynamic and Mobility Database
The next step is to load the database, which is MgAlCaSn.tdb in this example.
Different from the normal thermodynamic database, this database also
contains mobility model parameters for the phases of interest in addition to the
thermodynamic model parameters. Both are needed for carrying out
solidification simulation. By clicking the button on the toolbar, a pop-up
window as shown in Figure 7.5 will open, allowing user to select the database
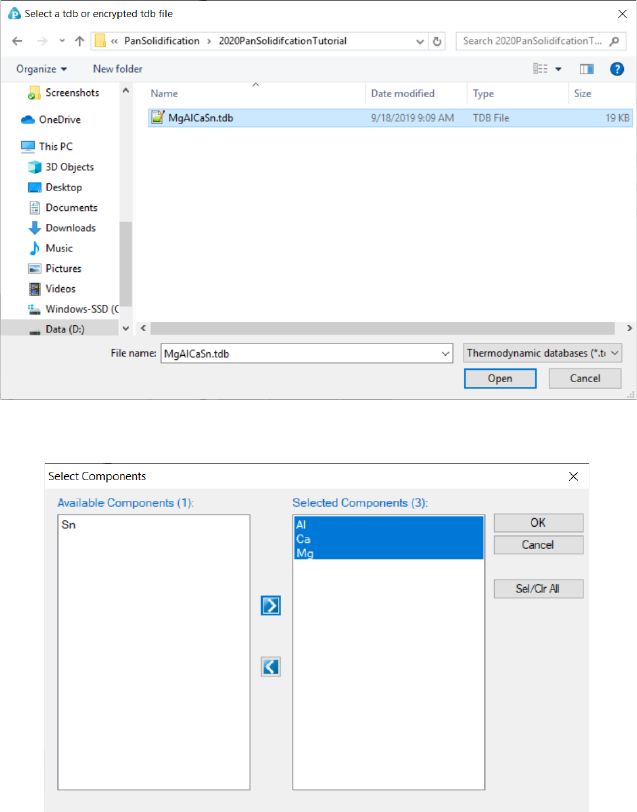
207
file. And Click “Open” to select the database, then a window as Figure 7.6 will
pop up for user to select components for PanSolidification simulation.
Figure 7.5 Dialog window for loading thermodynamic and mobility database.
Figure 7.6 Dialog window for components selection.
7.2.3 Step 3: Load Solidification Kinetic Database
A solidification kinetic database is required for solidification simulation. Such a
database contains kinetic parameters which are alloy dependent. To organize
these parameters in a more intuitive way, the standard XML format is adopted
and a set of well-formed tags are deliberately designed to define back diffusion
208
model for the morphology of primary phase (which could be plate, cylinder and
sphere) and its corresponding model parameters such as interfacial energy,
latent heat, coarsening geometric factor, dendrite tip factor, solute trapping
parameter, solid diffusivity factor and boundary layer factor.
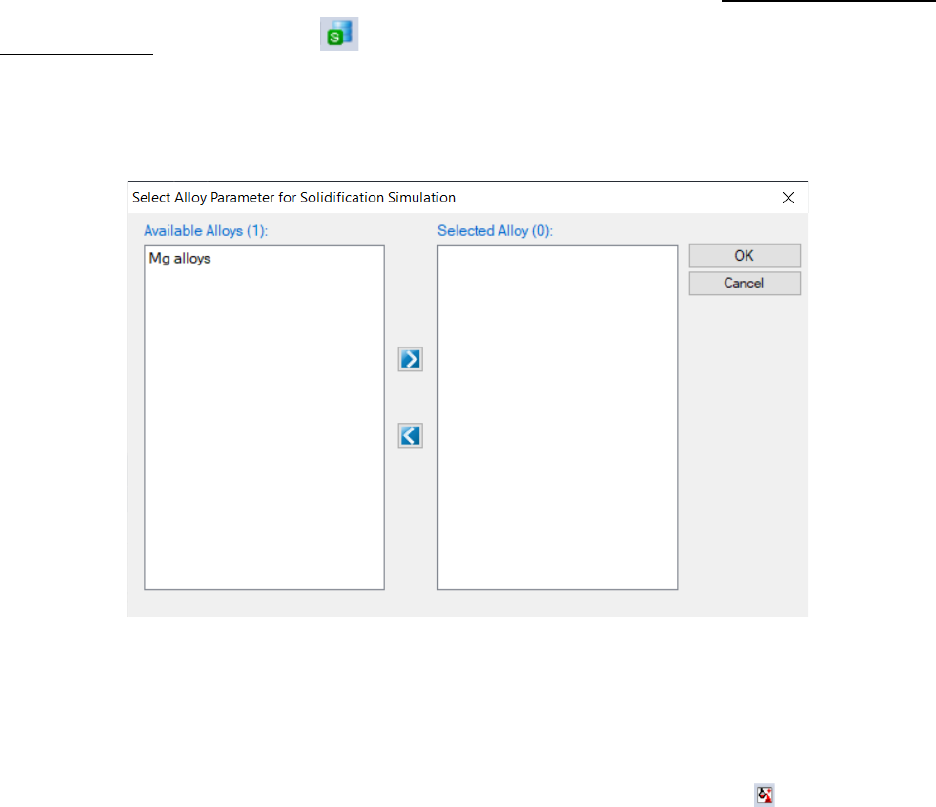
In this example, the MgAlloys.sdb is prepared. To load a solidification
database, user should navigate the command through menu PanSolidification
→ Load SDB, or click icon from the toolbar. After MgAlloys.sdb is chosen,
a dialog box pops out automatically for user to select the alloy for the
simulation. As shown in Figure 7.7, Mg alloys is contained in this SDB file.
Figure 7.7 Dialog box for selecting alloy parameter.
7.2.4 Step 4: Solidification Simulation
Perform solidification simulation through menu bar PanSolidification -
>Solidification Simulation with Back Diffusion or click icon from the tool
bar. A dialog box entitled “Solidification Simulation with Back Diffusion”, as
shown in Figure 7.8, pops out for user’s inputs to set up the simulation
conditions: alloy composition and solidification conditions. When setting the
solidification conditions, users need to be careful about the units used for the
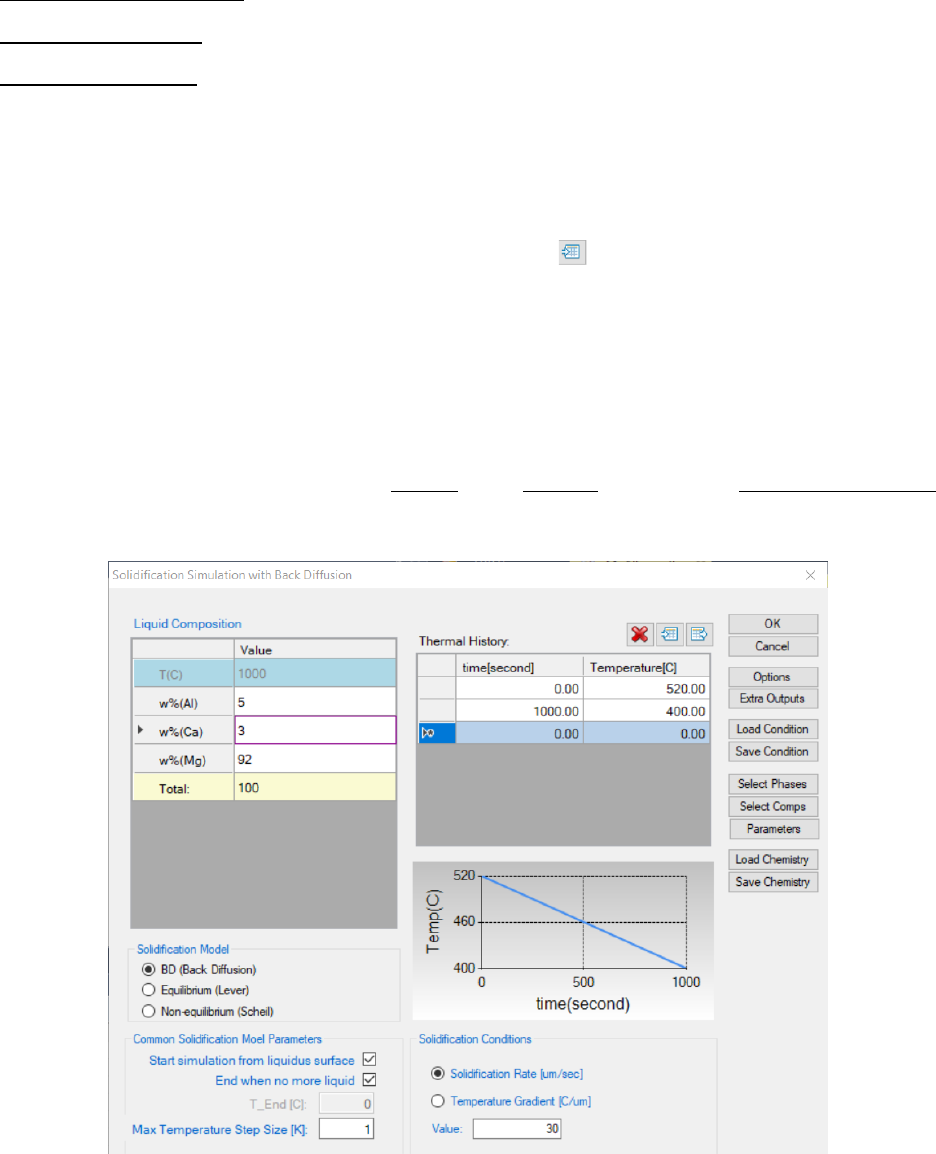
209
conditions. Click Option, a window as shown in Figure 7.9 will pops out for
units setting.
Alloy Composition: User can set alloy composition by typing in or use the
Load Chemistry function. User can also save the alloy composition through
Save Chemistry. This is especially useful when working on a multi-component
system, so that user does not need to type in the chemistry every time.
Solidification Conditions: The Cooling Rate of solidification can be defined
through Thermal History window. The cooling rate determined by cooling
curves can also be imported by click the icon as shown in Figure 7.8. The
Solidification Rate and Temperature Gradient can be defined from the
interface. As the Cooling Rate (CR), Solidification Rate (V) and Temperature
Gradient (G) has a relationship of CR = G*V, user may choose to provide either
Solidification Rate or Temperature Gradient.
User can also define the output Table and Graph within the Output Options
window.
210
Figure 7.8 Dialog box for setting solidification simulation conditions
Figure 7.9 Dialog box for setting units.
7.2.5 Step 5: Customize Simulation Results
As all other calculations available in Pandat
TM
, upon the completion of the
solidification simulation, a default table with related solidification related
properties (time, temperature, secondary dendrite arm spacing, solid and liquid
phase fractions, etc.) is automatically generated and a default graph for
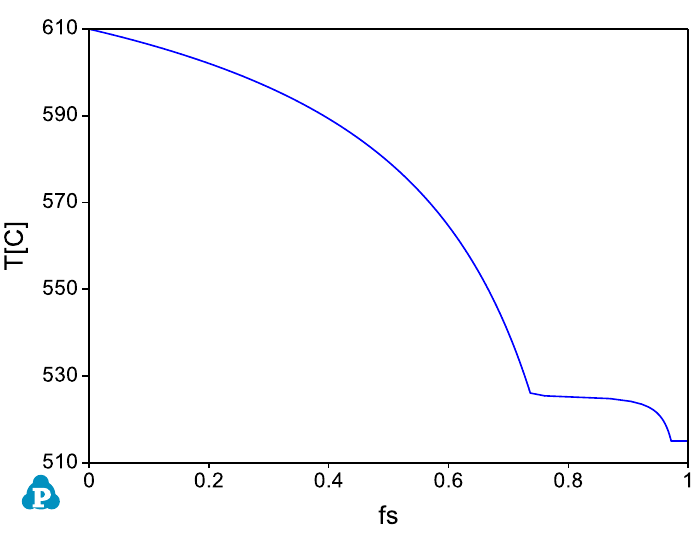
temperature (T) vs solid fraction (fs) is displayed as shown in Figure 7.10. User
can refer to sections 2.3 and 2.4 to learn how to customize simulated graph
and table.
211
Figure 7.10 Default graph plotting Temperature vs f
s
during solidification.